lithium-ion battery Working, Advantages, Disadvantages, Materials Used
Hello guys, welcome back to my blog. In this article, I will discuss what is a lithium-ion battery, the functions of the lithium-ion battery, the working of the lithium-ion battery, materials used in a lithium-ion battery, advantages, disadvantages, applications, and comparison of lithium-ion battery with other batteries.
If you have any electrical, electronics, and computer science doubts, then ask questions. You can also catch me on Instagram – CS Electrical & Electronics
Also, read:
- Top 17 CAN Protocol Questions Asked In An Interview With Answers
- Difference Between SRAM And DRAM, Characteristics, Advantages
- What Is Marx Generator, Working, Circuit Design, Applications
lithium-ion battery Working
At present, the lithium-ion battery is widely used in electric vehicles, laptops, mobiles, etc. The lithium-ion battery has lots of advantages compared to other batteries.
What is a lithium-ion battery?
The lithium-ion battery is made up of a combination of lithium cells. The lithium cell consists of some components such as a negative electrode, a positive electrode, electrolyte, separator, current collectors, etc. A cell means consists of a single anode and cathode separated by an electrolyte used to produce voltage and current.
Construction and working of lithium-ion cell
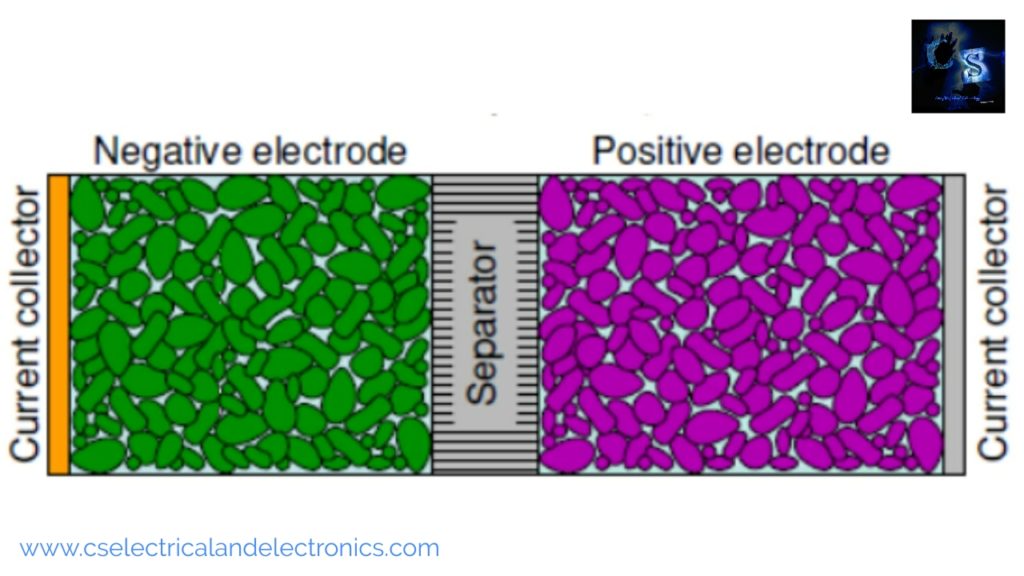
The fundamental components of an electrochemical cell are:
- Negative electrode
- Positive electrode
- Electrolyte
- Separator
- Current collectors
The function of the negative electrode
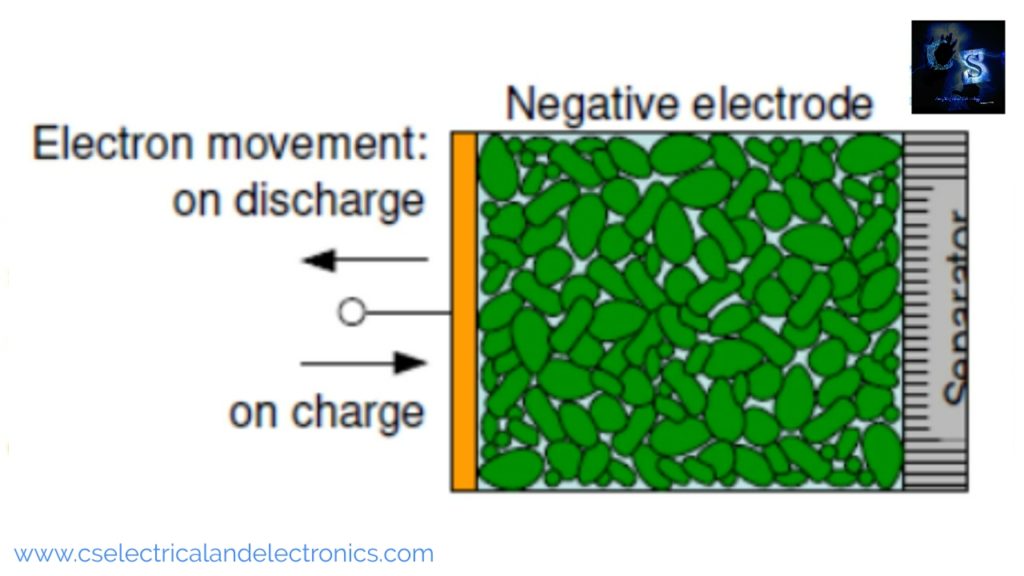
The above image shows the negative electrode. The negative electrode is often an alloy or metal or hydrogen. During the discharge process, the negative electrodes give up electrons to the external circuit. During the charging process, the negative electrode accepts electrons from the external circuit. During the discharge process, it is the anode but during the charging process, it is the cathode, but most people still call it anode.
The function of the positive electrode
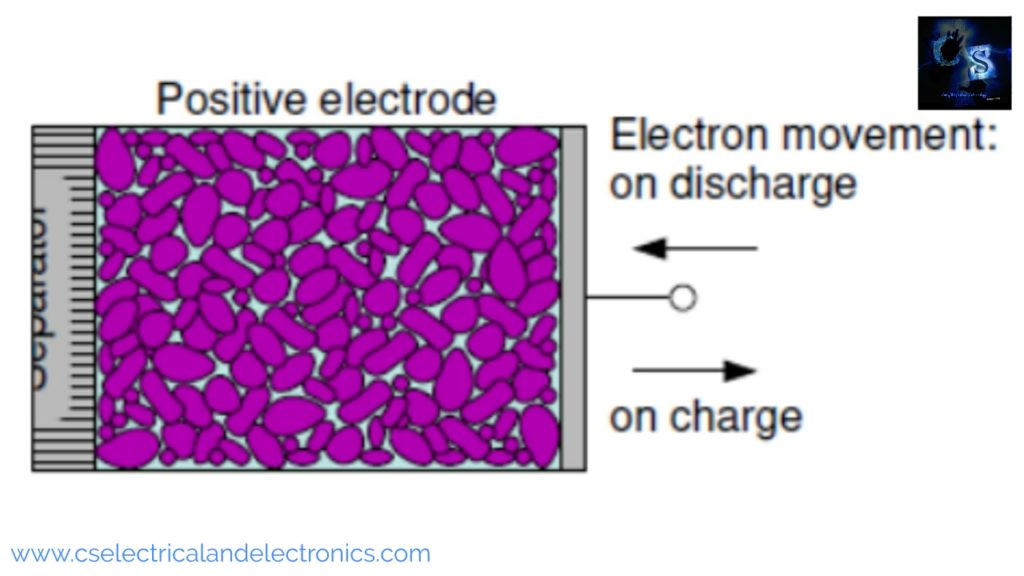
The above image shows the positive electrode. The positive electrode is often a sulfide, metallic oxide, or oxygen. During the discharge process, the positive electrode accepts electrons from the external circuit. During the charging process, the positive electrode gives up electrons to the external circuit. During discharge, the positive electrode becomes the cathode and technically, during the charging process, it becomes the anode, but most people still call it cathode.
The function of the electrolyte
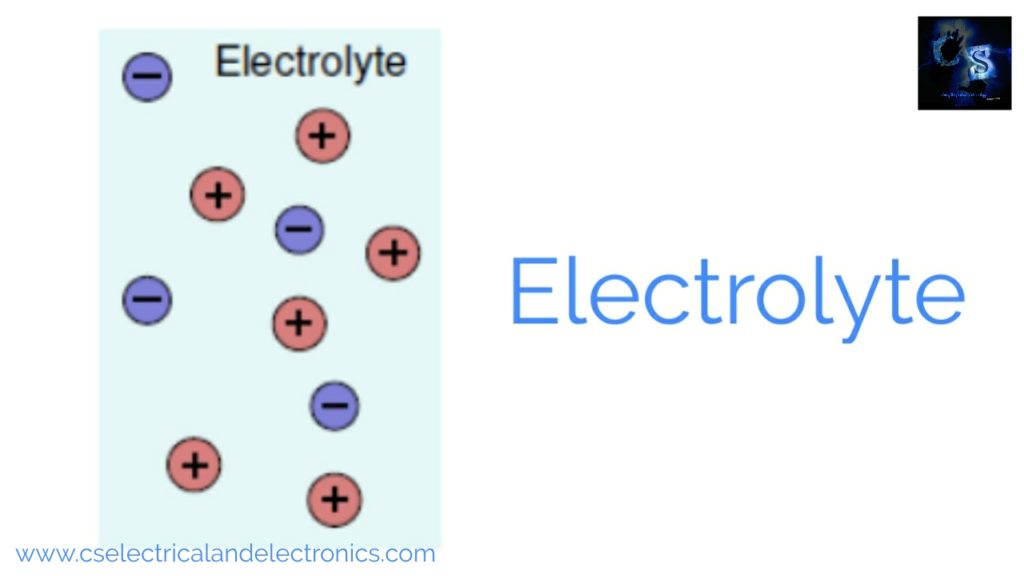
When the electrons move into the external circuit, compensating ions must move internally to the cell. Cations are ions with net positive charge as shown in the above image, during the discharging process they move through the electrolyte toward the positive electrode. Anions are ions with a net negative charge, during the discharging process, they move through the electrolyte toward the negative electrode.
The electrolyte provides a bath for internal ion charge to transfer between the electrodes. The electrolyte is often solvent containing dissolved chemicals such as base, acid, or salt providing ionic conductivity. The electrolyte must be an electronic insulator to avoid self-discharge.
The functions of a separator and current collectors
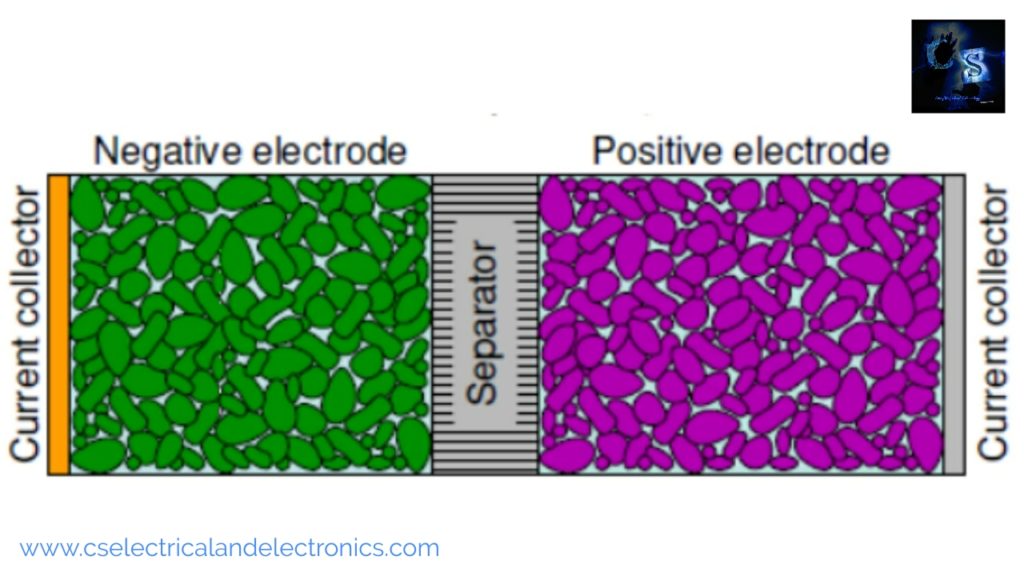
The function of the separator in the lithium-ion cell is to electrically isolate the positive and negative electrodes to avoid self-discharge of the cell and short circuit. The separator is often made from fiber, polyethylene, glass mat, or polymer. The function of the current collector is to conduct electrical current to cell terminals, it is made of metal foils.
The nominal voltage of the lithium-ion cell
The nominal voltage of the lithium-ion cell is 3.60V/cell.
Benefits or advantages of lithium-ion cell
- Lithium-ion cell has higher energy and power density when compared to other batteries.
- Lithium-ion cells self-discharge very slowly.
- The energy density of the lithium-ion cell is 400 wh/L.
- The energy efficiency of the lithium-ion cell is 80%.
- The power density of the lithium-ion cell is 800+ W/L.
Drawbacks or disadvantages of lithium-ion cell
- The cost of lithium cells is very high.
- A battery management system is required.
- Sensitive to overcharge leads to exposure.
- Lithium plating is generally caused at low temperatures or voltages, which causes loss in lithium.
Applications of the lithium-ion cell
The lithium-ion cells are used in;
- Defense
- Aerospace
- Instrumentation
- 04. Oil drilling
- 05. Mobile phones
- 06. Laptops
- 07. Power tools
- 08. Electric powertrains
I hope this article ” lithium-ion battery Working ” may help you all a lot. Thank you for reading ” lithium-ion battery Working “.
Also, read:
- 10 Tips To Maintain Battery For Long Life, Battery Maintainance
- 10 Tips To Save Electricity Bills, Save Money By Saving Electricity
- 100 (AI) Artificial Intelligence Applications In The Automotive Industry
- 100 + Electrical Engineering Projects For Students, Engineers
- 1000+ Automotive Interview Questions With Answers
- 1000+ Control System Quiz, Top MCQ On Control System
- 1000+ Electrical Machines Quiz, Top MCQs On Electrical Machines
- 1000+ MATLAB Simulink Projects For MTech, Engineering Students

