What Is Electrolyzer, Type, Working, Cost Of Electrolyzer
Hello guys, welcome back to my blog. In this article, I will discuss what is electrolyzer, the types of electrolyzer, its working, its cost, and the working of the Alkaline water electrolyzer.
If you have any electrical, electronics, and computer science doubts, then ask questions. You can also catch me on Instagram – CS Electrical & Electronics And Chetan Shidling.
Also, read:
- Types Of Hydrogen Storage That Can Be Used In The Future
- EV Charging Station Installation Costs With The TATA Power Franchise
- EV Charging Station Installation Costs With The TATA Power Franchise
What Is Electrolyzer
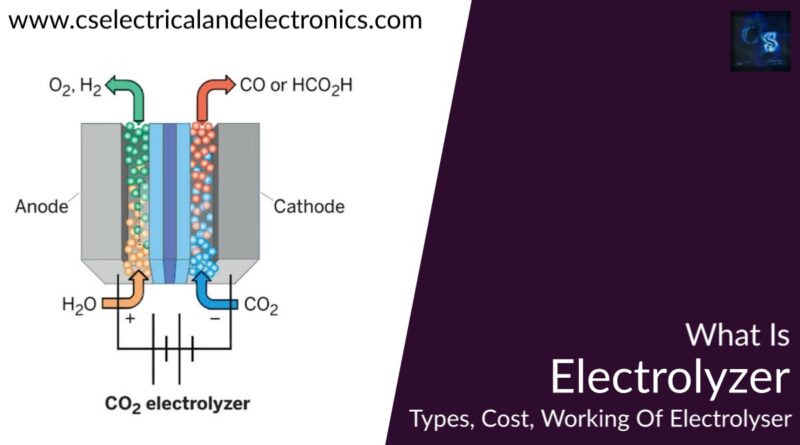
An electrolyzer transforms electricity into chemical energy which creates hydrogen. Hydrogen can be kept for instance in pressure tanks or any other metal hydrides. Hydrogen can be created from water electrolysis in different ways such as alkaline, acidic, and solar photo production. Alkaline and acidic water electrolyzers are commercially known today. Alkaline water electrolyzer will be the primary subject of interest in this dissertation and will be examined in particular in this section.
One of the considerable promising kinds of acidic electrolyzers is the proton (polymer) exchange membrane (PEM) electrolyzer, which corresponds to that of the PEM fuel cell described in section 3.2.1. Honestly, if appropriately designed, the exact PEM unit can be worked as an electrolyzer to create hydrogen, as well as a hydrogen-driven fuel cell to create electricity. The basic distinction between the PEM electrolyzer and the PEM fuel cell is that the hydrogen and oxygen reactions on the electrodes are opposing.
Thus, the electrolyzer delivers H2 at the cathode and O2 at the anode, while the fuel cell consumes H2 at the anode and O2 at the cathode. Furthermore, the decomposition of water into H2 and O2 in the electrolyzer is non-spontaneous and needs electric power for the reaction to happen, while the opposite fuel cell reaction is intuitive and delivers electric power.
In both circumstances, the reversible voltage is constant and identical to 1.23V. On the other hand, one can utilize the same equations to model PEM electrolyzers as to standard PEM fuel cells, only the model’s parameters might have other signs. The acquisition cost for a PEM electrolyzer is over 1000 euros/kW. The high cost of the elements is the primary drawback of this technology.
The solar photo production techniques are futuristic structures of hydrogen production. According to PV energy-driven water breaking systems, two critical systems should be evaluated for coming R&D. These methods are PV and electrolyzer systems and photoelectrochemical systems. From a useful point of view, the methods of most interest are the variety of PVs and electrolyzers.
This is expected to the relatively mature alkaline electrolyzer technology and the rapid growth of silicon photovoltaic technology. The photoelectrochemical systems have the benefit of connecting the PV generator and electrolyzer into one system without wires. A solar to hydrogen transformation efficiency with this method is approximately 15%.
Alkaline water electrolyzer types
Conventional Alkaline Water Electrolysis:
The electrolyte utilized in an alkaline water electrolyzer has lived aqueous potassium hydroxide (KOH), especially with solutions of 20-30 wt.%. This engagement range provides the optimal conductivity and erosion resistance of stainless steel. The standard working temperatures and forces of this electrolyzer are 70-100°C and 1-30 bar respectively.
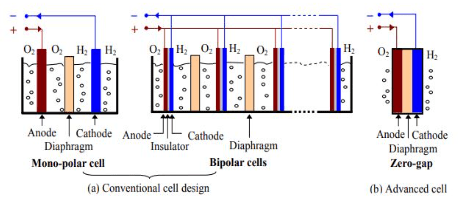
Physically an electrolyzer consists of various electrolytic cells, linked in parallel. Two different cell structures exist: mono-polar and bipolar. In mono-polar cells, the electrodes are either negative or positive while bipolar cells contain electrodes that are negative on one side and positive on the further side divided by an electrical insulator, as illustrated in Fig. 3.7a. The bipolar electrolyzer stacks are better compact than the mono-polar ones.
The bipolar electrolyzer works at higher pressures (up to 30 bar), and this is another benefit because it lowers the compression work needed to store the hydrogen created by the electrolyzer. Mono-polar systems work at atmospheric pressure. The benefit of the compactness of the bipolar cell structure shows shorter current paths in the electrical wires and electrodes compared to the mono-polar cell design. This decreases the failures due to the internal ohmic resistance of the electrolyte and thus increases the electrolyzer efficiency.
Nevertheless, there are some drawbacks of bipolar cells, for instance, a single cell loss leads to the malfunctioning of the entire stack. Mono-polar cells can be combined separately in the stack relying on the voltage-current demands, a single cell can be detached in the case of loss without seriously troubling performance. Again, the compactness and increased pressures of the bipolar electrolyzers need a relatively sophisticated and complicated system design. The fairly easy and studied mono-polar electrolyzers systems are in comparison less expensive to manufacture. However, most alkaline water electrolyzers manufactured today are bipolar.
Advanced Alkaline Water Electrolysis:
The primary purposes of the advanced water electrolyzers are: (1) to lower the practical cell voltages to lower the unit cost of electrical power and thereby the working costs; and (2) to improve the current density and thereby decrease the investment costs. Even these two purposes are conflicting with each other because expanding the current densities yield improves the cell voltage due to expanding the ohmic resistance as well as improving the over-voltages at the anodes and cathodes. In practice, three essential advancements of alkaline water electrolyzer are supposed to reach the two purposes stated above.
(1) Creating new cell configurations to decrease the surface-specific cell resistance despite improved current densities (e.g., zero-gap cells and low-resistance diaphragms).
(2) Processing at more elevated temperatures (up to 60°C) to improve electric conductivity of the electrolyte, i.e., to lower the electric cell resistance.
(3) Creating new electrocatalysts that lower anodic and cathodic over-voltages.
In the zero-gap cell design, as illustrated in Fig. 3.7b, the electrode materials are clustered on either side of the diaphragm so that the hydrogen and oxygen gasses are pushed to leave the electrodes at the end. Most manufacturers are already embracing this design. The acquisition cost of this class is approximately 500 euro/kW.
Alkaline water electrolyzer cost
A typical alkaline water electrolyzer, which creates the use of fossil fuel sources for electricity, is much more affordable than a renewable electrolyzer, which makes use of solar or wind power bases for electricity. Yet, the standard alkaline water electrolyzer mourns the environmental damages resulting from fossil fuel fuming in power plants and means very little improvement from the current energy regime. The capital costs of traditional alkaline water electrolyzers vary from 276 euro/kW to 568 euro/kW. For instance, a 2MW alkaline water electrolyzer with an efficiency of roughly 80% would cost roughly 568 euro/kW and the cost of hydrogen created is approximately 18 euro/GJ.
Although the environmental benefits of the renewable electrolyzer far surpass other methods, its use is not recommended in the short term. Renewable methods of electricity production are themselves budding technologies. The price of hydrogen production from renewable electrolysis is just high to be parsimonious. Yet, as a long-term goal, this presentation approach is particularly promising. The price of the electrolyzer stays reducing, as element materials persist to be modified and created.
Alkaline water electrolyzer operation and I-U characteristics
The deterioration of water into hydrogen and oxygen can be performed by handing a DC electric current between two electrodes divided by a KOH electrolyte with suitable ionic conductivity. Water is an extremely poor ionic conductor and for this cause, a conductive electrolyte must be utilized, so that the response can proceed at a technically adequate cell voltage. The half cell reactions and the general reaction are
Anodic half-reactions: 2OH– ⇒ 1/2O2 + H2 O + 2e–
Cathodic half-reaction: 2H2O ⇒ H2 + ½ O2
The overall reaction: H2O ⇒ H2 + 1/2O2
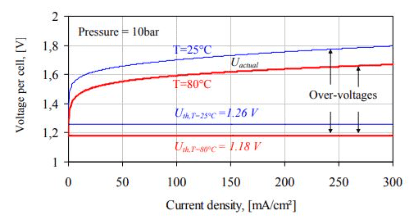
A plot of the academic and actual voltages (Uth, Uactual) for an alkaline water electrolyzer cell versus the present density at high and lower working temperatures is displayed in the below figure. The distinction between the two I-U curves is largely due to the temperature dependency of the overvoltages.
This was about “What Is Electrolyzer“. I hope this article may help you all a lot. Thank you for reading.
Also, read:
- 10 Tips To Maintain Battery For Long Life, Battery Maintainance
- 10 Tips To Save Electricity Bills, Save Money By Saving Electricity
- 100 (AI) Artificial Intelligence Applications In The Automotive Industry
- 100 + Electrical Engineering Projects For Students, Engineers
- 1000+ Control System Quiz, Top MCQ On Control System
- 1000+ Electrical Machines Quiz, Top MCQs On Electrical Machines
- 1000+ MATLAB Simulink Projects For MTech, Engineering Students
- 50 Tips To Save Electricity At Home, Shop, Industry, Office
Author Profile
- Chetu
- Interest's ~ Engineering | Entrepreneurship | Politics | History | Travelling | Content Writing | Technology | Cooking
Latest entries
 All PostsApril 19, 2024What Is Vector CANoe Tool, Why It Is Used In The Automotive Industry
All PostsApril 19, 2024What Is Vector CANoe Tool, Why It Is Used In The Automotive Industry All PostsApril 13, 2024What Is TCM, Transmission Control Module, Working, Purpose,
All PostsApril 13, 2024What Is TCM, Transmission Control Module, Working, Purpose, All PostsApril 12, 2024Top 100 HiL hardware in loop Interview Questions With Answers For Engineers
All PostsApril 12, 2024Top 100 HiL hardware in loop Interview Questions With Answers For Engineers All PostsMarch 22, 2024Driver Monitoring Systems In Vehicles, Working, Driver Sleepy Alert
All PostsMarch 22, 2024Driver Monitoring Systems In Vehicles, Working, Driver Sleepy Alert