Fuel Cell Working, Applications, Types, Advantages, Disadvantages
Hello guys, welcome back to my blog. In this article, I will discuss fuel cell working, diagram of it, applications, types of fuel cells, advantages, disadvantages of fuel cells, and comparison between fuel cells and batteries.
If you have any doubts related to electrical, electronics, and computer science, then ask questions. You can also catch me on Instagram – CS Electrical & Electronics And Chetan Shidling.
Also, read:
- Top 20+ Electric Vehicle Projects With MATLAB Simulink File In 2022
- SoC Estimation Using Extended Kalman Filter In MATLAB Simulink
- A Definitive Learner’s Guide For Electric Vehicle Technology Enthusiasts
Fuel Cell Working
Fuel cell approaches work without pollution when operated on pure hydrogen, the foremost by-products existing pure water and heat. When operating on hydrogen-rich reformate gas combinations, some toxic emissions result although they are smaller than those radiated by an internal combustion engine utilizing conventional fossil fuels. To be honest, internal combustion engines that combust lean mixtures of hydrogen and air also result in excessively low pollution levels that emanate primarily from the unexpected burning of lubricating oil.
Fuel cell systems appropriate for automotive applications work at low temperatures (typically smaller than 212ºF/100 ºC). This is a benefit in that the fuel cells need little warmup time, high-temperature risks are decreased, and the thermodynamic efficiency of the electrochemical reaction is naturally more useful. This is a drawback in that medium-grade waste heat is more challenging to eject(especially in hot climates) so cooling approaches must be larger, and the electrochemical reaction moves more slowly than at high temperatures. Reformers utilized in conjunction with fuel cells work at high temperatures and thus may need prolonged warmup periods.
Applications Of Fuel Cell
Fuel cell approaches can be utilized in co-generation applications. In addition to electrical power, fuel cells develop pure hot water and medium-grade heat, both of which can potentially be utilized in association with household or industrial applications. When this is accomplished, the overall efficiency of the combined approaches increases.
Fuel cells can be utilized in a broad range of applications, delivering power for applications across numerous sectors, including transport, industrial or commercial or residential facilities, and long-term power storage for the grid in reversible systems. Fuel cells have several advantages over traditional combustion-based technologies currently utilized in many power plants and vehicles.
Fuel cell systems do not need recharging. Instead, fuel cell approaches must be re-fueled, which is quicker than charging a battery pack and can deliver greater range relying on the size of the storage tank.
Advantages Of Fuel Cell
When utilized as an electrical energy-generating machine, fuel cells need fewer energy conversions than those compared with a heat engine. When employed as a mechanical energy-generating machine, fuel cells need an equal number of conversions, although the exact transformations are dissimilar.
Every energy conversion has an associate energy loss so the fewer conversions there are, the better will be the efficiency. Therefore fuel cells are more ideally fitted to applications that need electrical energy as the end product, instead of mechanical energy.
Disadvantages Of Fuel Cell
Hydrogen which is of such advantage environmentally when utilized in a fuel cell is also its greatest disadvantage in that it is challenging to manufacture and store. Current manufacturing methods are expensive and energy-intensive and usually derive yet from fossil fuels. An adequate hydrogen infrastructure has however to be installed.
Fuel cells that utilize proton exchange membranes should not dry out during service and must stay wet during storage. Tries to start or run these fuel cells under dry situations can lead to damage.
Fuel cells suited for automotive applications generally need the help of a platinum catalyst to enable the power generation reaction. Platinum is an infrequent metal and is extremely expensive.
Fuel Cell Working
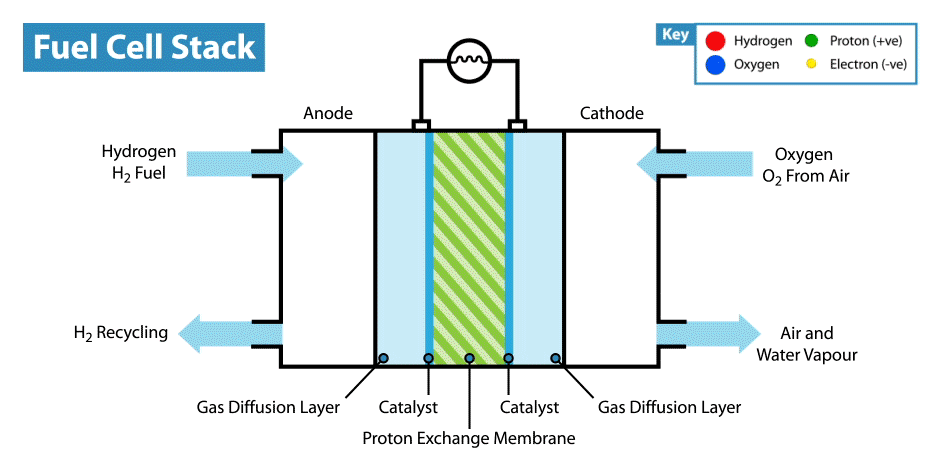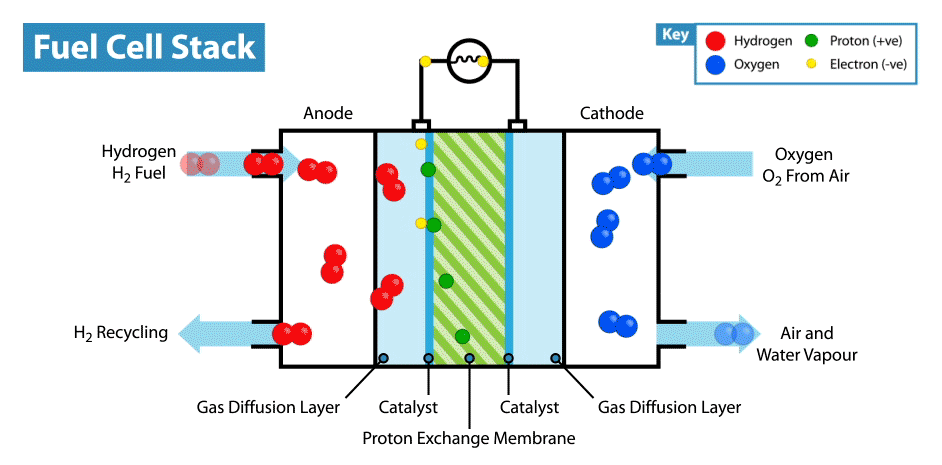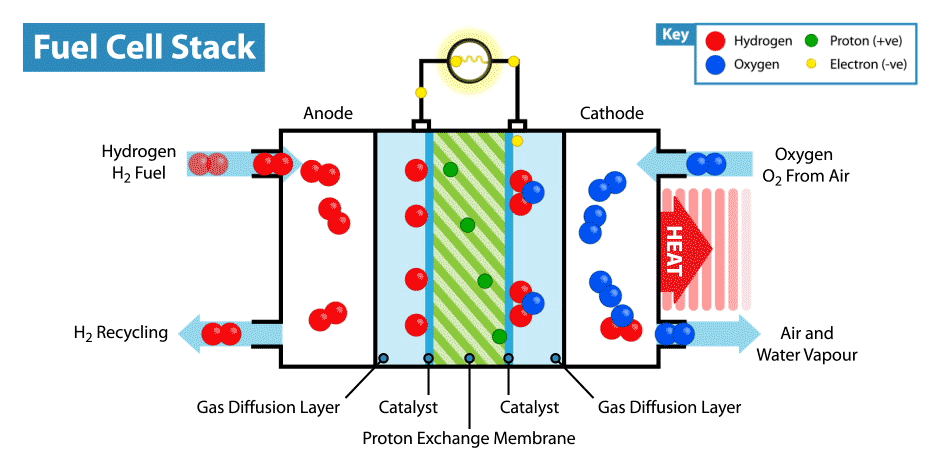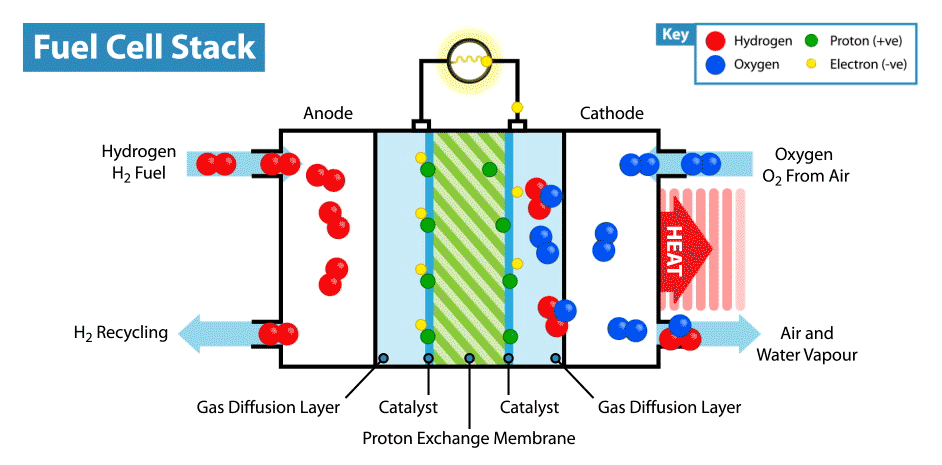
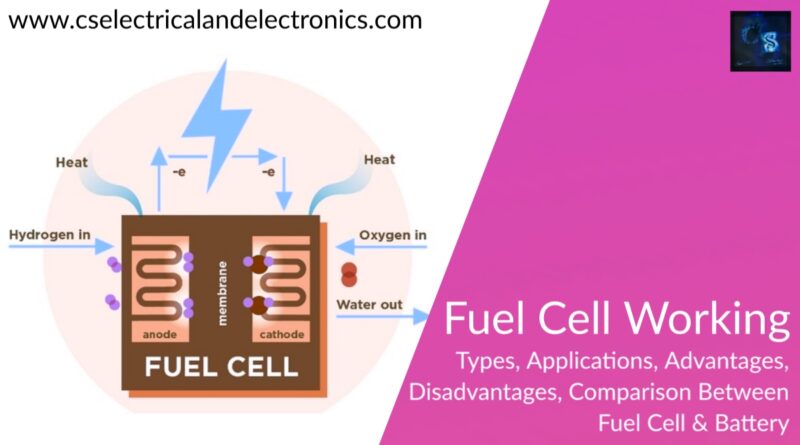
A fuel cell is an energy transformation instrument that transforms the chemical energy of fuel instantly into electricity without any medium thermal or mechanical processes. A primary characteristic of fuel cells is that the electric current load specifies the consumption rate of oxygen and hydrogen. In an actual approaches application, a type of electrical load may be applied to the fuel cell.
Fuel cells operate like batteries, though they do not drive down or require recharging. They deliver electricity and heat as long as fuel is provided. A fuel cell consists of two electrodes—an anode or negative electrode and a cathode or positive electrode—sandwiched around an electrolyte. A fuel, such as a hydrogen, is provided to the anode, and the air is provided to the cathode.
In a hydrogen fuel cell, a catalyst at the anode dissolves hydrogen molecules into electrons and protons, which take various approaches to the cathode. The electrons go via an external circuit, producing a flow of electricity. The protons migrate via the electrolyte to the cathode, where they combine with oxygen and the electrons to deliver water and heat.
Fuel Cells Vs Batteries
Fuel cells and batteries are both galvanic cells and thus have many likenesses. Both fuel cells and batteries have an anode and a cathode in connection with an electrolyte. Both of them generate electrical energy by transforming chemical energy from a high energy state to a lower energy state utilizing an electrochemical reaction.
These reactions happen at the anode and cathode with electron transfer moved via an external load in demand to complete the reaction. Particular cells of both batteries and fuel cells develop only small DC voltages, which are then connected in series to achieve significant voltage and power capacities.
Fuel cells vary from batteries in the disposition of their anode and cathode. In a battery, the cathode and anode are metals; zinc or lithium is commonly utilized for the anode and metallic oxides for the cathode. In a fuel cell, the anode and cathode are formed of gases constantly in contact with a platinum catalyst to enable the power generating reaction. Hydrogen or a hydrogen-rich gas mix is commonly utilized as the anode and oxygen or air as the cathode.
Types Of Fuel Cells
The most well-known high-temperature fuel cells are:
- Molten carbonate
- Solid oxide
The most well-known low-temperature fuel cells are:
- Alkaline
- Phosphoric acid
- Proton exchange membrane (or solid polymer)
This was all about “Fuel Cell Working“. I hope this article “Fuel Cell Working” may help you all a lot. Thank you for reading.
Also, read:
- 10 Tips To Maintain Battery For Long Life, Battery Maintainance
- 10 Tips To Save Electricity Bills, Save Money By Saving Electricity
- 100 (AI) Artificial Intelligence Applications In The Automotive Industry
- 100 + Electrical Engineering Projects For Students, Engineers
- 1000+ Control System Quiz, Top MCQ On Control System
- 1000+ Electrical Machines Quiz, Top MCQs On Electrical Machines
- 1000+ MATLAB Simulink Projects For MTech, Engineering Students
- 50 Tips To Save Electricity At Home, Shop, Industry, Office
Author Profile
- Chetu
- Interest's ~ Engineering | Entrepreneurship | Politics | History | Travelling | Content Writing | Technology | Cooking
Latest entries
 All PostsMay 9, 2024Top 5 Plus 5 Best Automotive Courses For Engineers From Udemy
All PostsMay 9, 2024Top 5 Plus 5 Best Automotive Courses For Engineers From Udemy All PostsApril 29, 2024Top 11 Free Courses On Battery For Engineers With Documents
All PostsApril 29, 2024Top 11 Free Courses On Battery For Engineers With Documents All PostsApril 19, 2024What Is Vector CANoe Tool, Why It Is Used In The Automotive Industry
All PostsApril 19, 2024What Is Vector CANoe Tool, Why It Is Used In The Automotive Industry All PostsApril 13, 2024What Is TCM, Transmission Control Module, Working, Purpose,
All PostsApril 13, 2024What Is TCM, Transmission Control Module, Working, Purpose,